Adeno-Associated Virus Antibodies and ELISA
Adeno-Associated Virus, AAV, a nonpathogenic virus species belonging to the Parvoviridae family. AAV is classified as small (25nm) containing a single-stranded nonenveloped DNA genome. Infection with AAV only occurs with the assistance of other viruses, for example herpesvirus or adenovirus (hence the name adeno-associated virus), causing only a very mild immune response in humans. There are twelve serotypes of human AAV but the number of nonhuman AAVs exceeds 100. AAV2 is the only mammalian DNA virus that is known to integrate in a specific site of the genome.
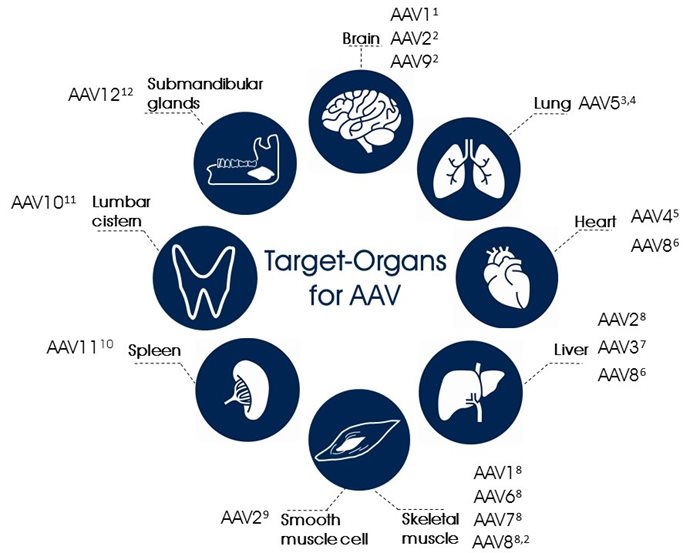
Immunogenicity is low and the ability to infect dividing as well as non-dividing cells with stable expression make the adeno-associated virus an appealing vector for the application in gene therapy. AAV has successfully been proven as gene therapy vector with the means to attach and enter the target cell, transfer to the nucleus and express the transgene in a stable manner over a sustained period of time. In several clinical trials (e.g. FIX, CFTR, Parkinsons’s, Canavan disease) AAV did not show any serious vector-related adverse effects. Clinically relevant tissues have, however, revealed a diversified susceptibility to AAV infection. The gene transduction with AAV2 vector in muscle, retina, liver and heart resulted in lower gene expression compared to AAV serotypes 1, 5, 8 and 9 transduction in the respective tissues. The challenge of using AAV as gene therapy vector, however, lies in the resistance of some tissues to transduction with the available AAV serotypes.
Target-Organs for AAV